Q. 1. How many elements were known when Mendeleev classified the elements?
Q. 2. Why are the elements in the same group of the periodic table show close resemblance in chemical behavior?
Q. 3. Why are anions bigger than their parent atoms?
Q. 4. How does the electronic configuration of an atom relate to its position in the Modern Periodic Table?
Q. 5. Write the demerits of Newlands’ law of octaves.
Q. 6. Write the merits of Mendeleev’s classification of elements.
Q. 7. In the following diagram for the first three periods of the periodic table, five elements have been represented by the letters a, b, c, d and e (which are not their chemical symbols ): 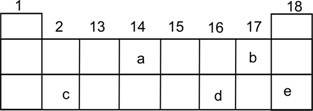
(i)Select the letter which represents a halogen.
(ii)Select the letter which represents a noble gas.
(iii)What type of bond is formed between a and b?
(iv)What type of bond is formed between c and d?
(v)Which element will form a divalent anion?
Q. 8. What are the limitations of Dobereiner’s triad?
Q. 9. What was the criterion used by Mendeleev in creating his periodic table?
Q. 10. State the modern periodic law.
Q. 11. Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the periodic table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Q. 12. Element X forms a chloride XCl2, which is a solid with a high melting point. X would most likely be in the same group of the periodic table as:
(a) Na (b) Mg (c) Al (d) Si
Q. 13. For each the following triads, name the element with the characteristics specified below:
| Elements | Least atomic radius | Chemically L least reactive |
| F, Cl, Br | ………….. |
…………… |
| Li, Na, K | …………… |
……………. |