AMINES
Question 1: Classify the following amines as primary, secondary or tertiary:
(i) 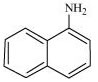 (ii)
(ii) 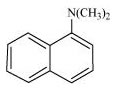
(iii) (C2H5)2CHNH2 (iv) (C2H5)2NH
Answer :
Primary: (i) and (iii)
Secondary: (iv)
Tertiary: (ii)
Question 2: (i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
Answer :
(i), (ii) The structures and their IUPAC names of different isomeric amines corresponding to the molecular formula, C4H11N are given below:
(a) CH3-CH2-CH2-CH2-NH2
Butanamine (10)
(b) 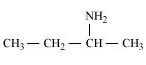
Butan-2-amine (10)
(c) 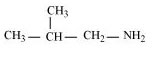
2-Methylpropanamine (10)
(d) 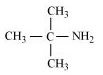
2-Methylpropan-2-amine (10)
(e) CH3-CH2-CH2-NH-CH3
N-Methylpropanamine (20)
(f) CH3-CH2-NH-CH2-CH3
N-Ethylethanamine (20)
(g) 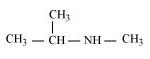
N-Methylpropan-2-amine (20)
(h) 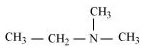
N,N-Dimethylethanamine (3°)
iii) The pairs (a) and (b) and (e) and (g) exhibit position isomerism.
The pairs (a) and (c); (a) and (d); (b) and (c); (b) and (d) exhibit chain isomerism.
The pairs (e) and (f) and (f) and (g) exhibit metamerism.
All primary amines exhibit functional isomerism with secondary and tertiary amines and vice-versa.
Question 3: How will you convert?
(i) Benzene into aniline
(ii) Benzene into N, N-dimethylaniline
(iii) Cl−(CH2)4−Cl into hexan-1, 6-diamine?
Answer :
(i) 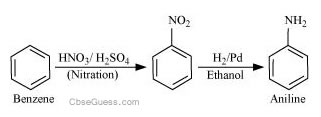
(ii) 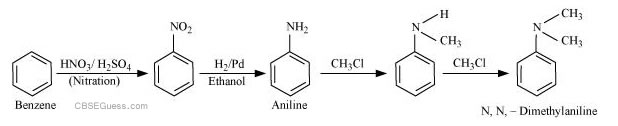
(iii) 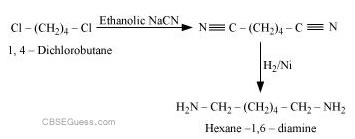
Prepared By: Mr. MANISH TULI
mail to: [email protected]