Carbon and Its Compounds
- Graphite - It is a grayish black opaque substance , lighter than diamond, soft and slippery to touch, conducts electricity, burns on strong burning to give CO2 .
- The chemical properties of both diamond and graphite are same as both burn in oxygen to give carbon dioxide. However, their physical properties are different due to difference in their structures ( different arrangements of carbon atoms).
Structure of Graphite
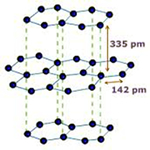
Each graphite crystal consists of layers of carbon atoms. Each carbon atom is joined to three other carbon atoms by strong covalent bonds to form flat hexagonal rings. The various layers of carbon atoms are held together by weak Van der Waals forces.
- Due to sheet like structure it is a soft substance due to which it is used as a dry lubricant fro machine parts.
- It is a good conductor of electricity--- Each carbon atom is joined to only three other carbon atoms by covalent bonds i.e. only 3 atoms are used in bond formation leaving the 4th atom to move freely. Due to this free electron , it conducts electricity.
Uses of Graphite –
- As lubricant for fast moving parts of machinery.
- For making carbon electrodes in dry cells and electric arcs. (The black coloured anode of a dry cell is made of graphite)
- For making pencil leads and black paints.
Buckminsterfullerene -- C60
It is an allotrope containing 60 carbon atoms joined together to form spherical molecules.
It is a spherical molecule with atoms arranged in interlocking hexagonal and pentagonal rings . There are twenty hexagons and twelve pentagons of carbon atoms in one molecule.
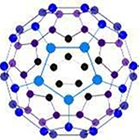
It is a dark solid at room temp. , burns to give CO2 , neither hard nor soft.
ORGANIC COMPOUNDS
The compounds of carbon are known as organic compounds as they contain hydrogen and many organic compounds containing oxygen or other elements. So, most organic compounds are HYDROCARBONS or their derivatives. They are covalent compounds having low melting and boiling points.
- Oxides of carbon , carbonates, hydrogencarbonates and carbides are also carbon compounds but they are not considered as organic compounds because their properties are different from those of common organic compounds.
Two properties of carbon –
- Catenation- atoms join with one another to form long chains .
3 types of chains ---
i) - Straight chain 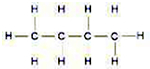
ii) - Branched chain 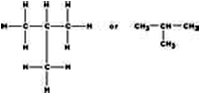
iii) - Closed chain 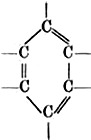
- Tetravalent –
Types of Organic compounds -
- Hydrocarbons
- Haloalkanes
- Alcohols
- Aldehydes
- Ketones
- Carboxylic acids ( Organic acids)
Submitted By Mrs. Kritika Bhola
Email Id : [email protected]




