AIEEE 2010 Chemistry
Q. 3. The time for half life period of a certain reaction A  Products is 1 hsour. When the initial concentration of the reactant ‘A’, is 2.0 mol L-1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L-1 if it is a zero order reaction?
Products is 1 hsour. When the initial concentration of the reactant ‘A’, is 2.0 mol L-1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L-1 if it is a zero order reaction?
Sol:
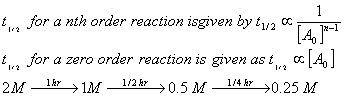
Answer: 3
Q. 4. The standard enthalpy of formation of NH3 is – 46.0 kJ mol-1. If the enthalpy of formation of H2 from its atoms is – 436 kJ mol-1 and that of N2 is – 712 kJ mol-1, the average bond enthalpy of N–H bond in NH3 is
Sol :
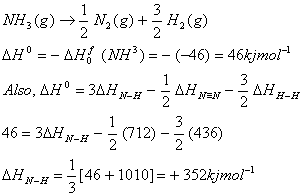
Answer: 2