AIEEE 2010 Chemistry
Note: Questions with (*) mark are from syllabus of class XI.
Q.1. A solution containing 2.675 g of CoCl3.6NH3 (molar mass = 267.5 g mol-1) is passed through a cation exchanger. The chloride ions obtained in solution were treated with excess of AgNO3 to give 4.78 g of AgCl (molar mass = 143.5 g mol-1). The formula of the complex is (Atomic mass of Ag = 108 u)
Sol :
Number of moles of the complex 
Number of moles of AgCl formed 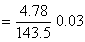
 Number of moles of AgCl formed = 3 × Number of moles of the complex
Number of moles of AgCl formed = 3 × Number of moles of the complex
 The formula of the complex is [Co(NH3)6]Cl3
The formula of the complex is [Co(NH3)6]Cl3
Answer: 1
Q. 2. If 10-4 dm3of water is introduced into 1.0 dm3 flask at 300 K, how many moles of water are in the vapour phase when equilibrium is established? (Given: Vapour pressure of H2O at 300 K is 3170 Pa ; R = 8.314 JK-1 mol-1)
Sol :
The volume occupied by water molecules in vapour phase is (1 – 10-4) dm3, that is approximately 1 dm3.
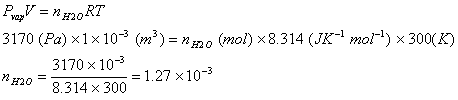
Answer: 4