![]()
CBSE Guess > Papers > Important Questions > Class X > Science > Physics > Rate of Chemical reactions By ; Mr. Nagesh
Physics (Rate of Chemical Reactions)
1. Rate of reaction:
- In a particular reaction, the concentration of chlorine produced changes from .0006 mol/litre to .003mol/litre in 200sec. Calculate average rate of the reaction.
- Why is rate of reaction in terms of reactants written negative?
- What do you understand by Rate of reaction?
- What do you mean by average and instantaneous rate of reaction? Give an example of instantaneous reactions.
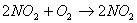 What happens to rate of reaction when 1) 2moles of oxygen are taken 2) 4 moles of NO2 are taken?
What happens to rate of reaction when 1) 2moles of oxygen are taken 2) 4 moles of NO2 are taken?- What is the effect of an increase in concentration on the rate of chemical reaction?
- A solution contains 0.5M of HCl in 2.5 litres of solution. Calculate its Molarity.
- Write the expression for the equilibrium constant of the following reaction
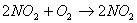
- Write the equilibrium constant for
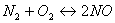 , what does the very small value of
, what does the very small value of 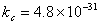 for this reaction indicates.
for this reaction indicates. - Why a large increase in temperature does slow down a reaction catalyzed by an enzyme?
- Which of the following are a slow and a fast reaction 1) setting of cement 2) formation of coal in earth’s crust?
- What is the effect of increase in temperature on rate of chemical reaction?
- Draw the graphs showing variation in rate of reaction with temperature for an explosive reaction, normal reaction and enzyme catalyzed reaction.
- Biological reactions in our body are controlled by biocatalysts. What name is given to these catalysts? How is their activity influenced by changes in temperature? Draw the graph for this variation.
- When a reversible chemical reaction does reaches a state of equilibrium? Write expression for the equilibrium constant (K) for the equilibrium reaction:
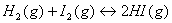 How will the numerical value of equilibrium constant change if the equation is written:
How will the numerical value of equilibrium constant change if the equation is written: 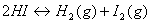 .
.
2. Acids and Bases:
- Give one example of strong and weak electrolyte.
- What are negative catalysts? Give an example.
- An aqueous solution has hydrogen ion concentration
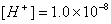 . Determine the pH of the solution. Will the solution be acidic or basic? Will the pH of above solution increase/decrease on adding a drop of 1M HCl. justify your answer?
. Determine the pH of the solution. Will the solution be acidic or basic? Will the pH of above solution increase/decrease on adding a drop of 1M HCl. justify your answer? - What is a catalyst? What happens to the catalyst after the chemical reaction is over?
- What was Arrhenius theory of acids and Bases? What is the major draw back of Arrhenius theory?
- State the protonic concept for acid and bases?
- State Lewis concept of acid and bases.
- Give an example of photochemical reaction.
- Choose a strong acid, weak acid, strong base and weak base:
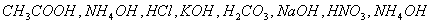
- The pH of an aqueous solution decreases from 3 to 2. Calculate how many times the hydrogen ion concentration of the solution will change.
- State and explain Le-Chatleirs principle between water and vapor be achieved in an open container.
- What do you mean by Dynamic equilibrium? Give one example. With the help of graph show the attainment of equilibrium in a chemical reaction.
| Physics Links | |
| 1. | Light |
| 2. | Optical Instruments |
| 3. | Nuclear Fission and Fusion |
| 4. | Universe |
| 5. | Electricity |
| 6. | Sources of energy |
| 7. | Rate of Chemical reactions |
