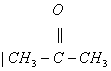AIPMT Examination
AIPMT Solved / Unsolved Papers
AIPMT Important Links
AIPMT 2007 Chemistry Screening
Q. 1. The correct order of ![]()
Sol. The correct order of ![]()
Correct choice: (1)
Q. 2. Which one of the following ionic species has the greatest proton affinity to form stable compound?
- I-
- HS-
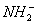
- E-
Sol. Strongest base would have the highest proton affinity i.e, ![]() Correct choice: (3)
Correct choice: (3)
Q. 3. In which of the following the hydration energy is higher than the lattice energy?
- SrSO4
- BaSO4
- MgSO4
- RaSO4
Sol. MgSO4 is the most soluble out of the given alkaline earth metal sulphates. Correct choice: (3)
Q. 4. Which of the following statements, about the advantage of roasting sulphide ore before reduction is not true?
- Roasting of the sulphide to the oxide is thermodynamically feasible.
- Carbon and hydrogen are suitable reducing agents for metal sulphides.
- The
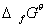 of the sulphide is greater than those for CS2 andH2S
of the sulphide is greater than those for CS2 andH2S - The
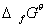 is negative for roasting of sulphide ore to oxide
is negative for roasting of sulphide ore to oxide
Sol. Carbon and hydrogen are not suitable for reduction of sulphides directly. Correct choice: (2)
Q. 5. The correct order of increasing thermal stability of
![]()
Sol. The correct order is ![]()
Correct choice: (3)
Q. 6. Sulphides ores of metals are usually concentrated by Froth Flotation process. Which one of the following sulphides oresoffers an exception and is concentrated by chemical leaching?
- Sphalerite
- Argentite
- Galena
- Copper pyrite
Sol. Argentite ore is leached with NaCN during extraction of silver in the Mc Arthur Forrest Cyanide process. Correct choice: (2)
Q. 7. Which one of the following anions is present in the chain structure of silicates?
Sol. Chain silicates have the general formula ![]() Correct choice: (4)
Correct choice: (4)
Q. 8. Which one of the following orders correctly represents the increasing acid strengths of the given acids?
Sol. The correct order is ![]()
Correct choice: (2)
Q. 9. Which of the following oxidation states are the most characteristic for lead and tin respectively?
- + 2, + 2
- + 4, + 2
- + 2, + 4
- + 4, + 4
Sol. Among common characteristic states for Pb and Sn, we find +2 and +4 respectively. Correct choice: (3)
Q. 10. Identify the incorrect statement among the following:
- Shielding power of 4f electrons is quite weak
- There is a decrease in the radii of the atoms or ions as one proceeds from La to Lu
- Lanthanoid contraction is the accumulation of successive shrinkages
- As a result of lanthanoid contraction, the properties of 4d series of the transition elements have no similarities with the 5d series of elements
Sol. The atomic radii of 4d and 5d elements down the group become quite similar due to lanthanidecontraction. Correct choice: (4)
Q. 11. Which one of the following ions is the most stable in aqueous solution?
(Atomic number. Ti = 22, V = 23, Cr = 24, Mn = 25)
Sol . ![]() is the most stable ion in aqueous medium. Correct choice: (2)
is the most stable ion in aqueous medium. Correct choice: (2)
Q. 12. The d electron configurations of Cr . Which one of the
following aqua complexes will exhibit the minimum paramagnetic behaviour?
![]()
Sol. ![]() will have two unpaired electrons and will show least paramagnetic character. Correct choice: (4)
will have two unpaired electrons and will show least paramagnetic character. Correct choice: (4)
Q. 13. Which of the following will give a pair of enantiomorphs?
Sol . The complex ion ![]() can show optical isomerism in its cis-isomer, and will form a pair of enantiomorphs. Its trans-form will be optically inactive (meso). Correct choice: (4)
can show optical isomerism in its cis-isomer, and will form a pair of enantiomorphs. Its trans-form will be optically inactive (meso). Correct choice: (4)
Q. 14. If NaCl is doped with ![]()
Sol. Number of moles of cationic vacancies
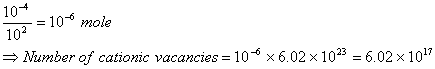 Correct choice: (4)
Correct choice: (4)
Q. 15. Which of the following presents the correct order of the acidity in the given compounds?
Sol.![]() Correct choice: (1)
Correct choice: (1)
Q. 16. The product formed in Aldol condensation is
- an alpha, beta unsaturated ester
- a beta-hydroxy acid
- a beta-hydroxy aldehyde or a beta-hydroxy ketone
- an alpha-hydroxy aldehyde or ketone
Sol. Aldol condensation leads to formation of ![]()
Correct choice: (3)
Q. 17. Reduction of aldehydes and ketones into hydrocarbons using zinc amalgam and conc.HCl is called
- Wolf-Kishner Reduction
- Clemmensen Reduction
- Cope Reduction
- Dow Reduction
Sol. This is Clemmensen’s reduction. Correct choice: (2)
Q. 18. Consider the following compounds
The correct decreasing order of their reactivity towards hydrolysis is
- (b) > (d) > (a) > (c)
- (b) > (d) > (c) > (a)
- (a) > (b) > (c) > (d)
- (d) > (b) > (a) > (c)
Sol. The attack of the nucleophile onto the carbonyl carbon is the rate-determining step. So, order must be (b) > (d) > (a) > (c) Correct choice: (1)
Q. 19. Which one of the following on treatment with 50% aqueous sodium
Sol. Benzaldehyde has no ![]() hydrogen atom, so it can undergo Cannizaro reaction. Correct choice: (3)
hydrogen atom, so it can undergo Cannizaro reaction. Correct choice: (3)
Q. 20. Which one of the following on reduction with lithium aluminium hydride yields a secondary amine?
- Methyl Cyanide
- Nitroethane
- Methylisocyanide
- Acetamide
Sol. Methyl isocyanide on reduction with LiAlH 4 will give dimethylamine. Correct choice: (3)