CBSE Guess > Papers > Important Questions > Class X > 2013 > Chemistry > Chemistry By Mr. Atul Kumar Gaur
CHEMISTRY FOR CBSE CLASS X
 
Sub-Chemistry
- Account for the following
- Elements C, N, O and F are all placed in the second period in the periodic table.
- Elements of group 17 are monovalent.
-
- How does Atomic Radius change as we more from left to right in a period ?
- The positions of three Elements P, Q and R in the Periodic table are shown below
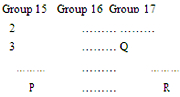
Which one of the three elements is most non - metallic ?
-
- On dropping a small piece of Sodium into an organic compound “A” with molecular formula C2H6O in a test tube a brisk effervescence is observed. On bringing a burning splinter the gas evolved burns with a pop sound. Identify ‘A’ and write the chemical equation.
- What will happen when you heat the organic compound ‘A’ at 443K with excess of concentrated Sulphuric acid ?
-
- Examine Elements of the third Period : Na, Mg, Al, Si, P, S, Cl, Ar and answer the following :
Choose
(i) Metals and
(ii) Non - Metals out of these elements.
- On which side of the Periodic table can we locate
(i) Metals and
(ii) Non-Metals?
Name the Metalloid out of the elements given above. Where are they located in the periodic table ?
- Name the functional group present in each of the following compounds.
C3H7OH
- State Modern period law. How many groups and periods are present in modern periodic table ?
-
- List two medicinal use of ethanol.
- What happens when ethanol is heated with excess of conc. H2SO4 at 443K (Give chemical equation) ? What role does conc. H2SO4 play in this reaction ?
- Give reasons for the following :
- Lithium atom is smaller than Sodium atom
- Chlorine (Atomic Number 17) is more electronegative than Sulphur (Atomic Number 16)
-
- State modern periodic law.
- State the place of metalloids in the periodic table.
- Three elements A, B and C have atomic number 7, 8 and 9 respectively.
- What would be their positions in the modern periodic table (Mention group and period both)
- Arrange A, B and C in the decreasing order of their size.
- Which one of the three elements is most reactive and why ?
-
- Draw the electron dot structure of -
- C2H2
- C2H5OH
- What are hydrogenation reactions ? Give an example.
- Draw the structure of the simplest ketone.
-
- State Modern Periodic Law.
- Why is position assigned to hydrogen in Periodic Table considered anomalous ?
- Account for the following :
- Elements of group 18 are called zero valent.
- Elements in a group of periodic table have similar chemical properties.
-
- Define the term Functional group. Identify the functional group present in the
following compounds :
- CH3 2CH2 2CH2 2OH
- -
- What will you observe on adding a 5% alkaline potassium permanganate solution
drop by drop to some warm ethanol taken in a test tube ? Write the name of the
compound formed during the above chemical reaction.
- Given below are four elements with their atomic numbers
-
| Element |
Atomic number |
| A |
16 |
| B |
11 |
| C |
3 |
| D |
14 |
- Identify the elements which belong to the same group of the Modern Periodic table.
- Arrange the given elements in decreasing order of atomic size.
- Write the formula of the oxide of B.
- Which of the above elements is a metalloid ?
 
Submitted By - Mr. Atul Kumar Gaur
JAIN INTERNATIONAL, SCHOOL KANPUR
Email Id : [email protected] |
|