![]()
CBSE Guess > Papers > Important Questions > Class XII > 2007 > Chemistry > Practice Paper 1
Practice Paper 1
- What is The Maximum Number Of Unpaired Electrons In Cu + [z = 29] Ions
- Why is Frankel defect not found in pure alkali metal hallides
- Why does a dry cell become dead after a long time even if it has not been used?
- What does reciprocal of gold number indicate.
- How is Xe of 2 prepared? Give example?
- Draw the structure of p4O10 and identify the number of single and double P-O Bounds.
- Write the IUPAC name of the compound [Cr(NH3)5(NC5)] [Zncl4].
- Why are corvohydrates generally optically active?
- Why G is positive for photochemical reaction?
- Name the chemical responsible for antiseptic properties of detol?
- Write the molecular orbital electron distribution of Oxygen. Specify its bond order magnetic property?
- A compound of vanadium has a magnetic moment of 1.73 B. M. work out the electronic configuration of the vanadium ion in the compound.
- Sodium Chloride solution freezs at lower tempreture than water boils at higher
Tempreture than water but boils at higher tempreture than water. Explain - Two liquid A and B form an ideal solution and have equal mole fraction if the Pure pressure of liquid A is 500 and of B is 300 mm Hg calculate the pressure of solution.
- Calculate the freezing point of an aqueous solution of an non eletrolyte having an a osmotic pressure of 2 atm at 300k (k = 1.86km-1 ; R = 0.082l atm.km)
- Why is entropy of substance taken as zero at absolute zero temperature?
- What pressure of H2 would be require to make the emf of hydrogen electrode. Zero in pure water at 25*C?
- How many hour does it take to reduce 3mol of Fe3+ to Fe2+ with 2A current?
- Give one test to distuinguish whether the given emultion is in water type of water in oil type emultion?
- What are the Interhalogen compound? How are they prepared? Why are most of the Interhalogen
Compound mor reactive then haloogen. - How many stereoisomers are possible forbut-2,3-diol. Draw these.
- Why is the boiling point of the glycol is high .
- What are the co- polymers. Give chemical for the preparation of cerylene?
- Are functioning system of antibodies present in new born babies?
- Is a diet consisting mainly of rice of adequate diet? Why or why not?
- In the formula Fe(X5 –C5 H5)2 what dose the prefix X5 indicate?
- What propellant is planned to be used in PLSV rocket?
- The composition of sample of wustite is Fe.93 O1.What percentage of Iron is Present in the form of Fe3+?
- Estimate the average S –F bond energy in SF6.The standered heat of formation values of SF6(g) and F(g) are –1100, 275& 875KJ/mol resp.
- The rate of constant for an isomerisation reaction A --> B is 4.5 x 10-3 /min. If the initial concentration of A is m, calculate the rate of reaction after 1 hour .
- Write the chemical equations for the extraction of phosphorus from calcium. Phosphate and discuss its allotrop.
- After an hour , the amount of certain radioactive substance disintegrated was 15/16th of the original amount. What is the half life of the radioactive substance?
- How are cynides and isocynide obtained from alkyle halide? Compare their solubility in water.
- (a) Give an account of extraction of silver from one of its ore?
(b) Zn2+ salt are white while Cu2+ salt are blue ?
(c) Give name three important halide of murcury and give one method of preparat- -ion of each? - Complete the reaction
(a) (CH3) CCH(OH) ——CH3—— H+——>
(b)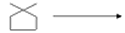
(c) Ph CH = CHen(OH) CH3 ———————> PBr3
(d) How you convert ethanol to lactic acid? - [a] Write down the ground state electronic configuration of F2+
[b] The kinetic energy of a sub-atomic particle is 4.55X10-25 J Calcu late the frequency of the particle wave - Calculate the E.M.F. and dG of cell reaction for the following cell at 25 C
Mg(s) |Mg2+(.001M) || Cu2+ | Cu(s)
E' Mg(s)|Mg2+ =-2.37 V E'Cu2+|Cu(s) =.34 V
F= 96500 C/mol
How long will it take an electric current of 0.15 A to deposit all the coper from 500 ml of 0.150 M copper sulfate solution? - [i] Explain why do transition elements show variable oxidation states. Write all possible oxidation states on an element having atomic no. 23
[ii] Describe the prepration of Potassium permagnate from pyrolusite ore.
- Atomic Structure and Chemical Bonding
- Solid State
- Solutions
- Thermodynamics
- Electrochemistry
- Chemical Kinetics
- P Block Elements
- D and F Block Elements
- Nuclear Chemistry
- Stereo Chemistry
- Organic Functional Group I
- Organic Functional Group II and III
- Surface Chemistry
- Chemistry In Every Day Life
- Polymers
- Practice Paper No.1
- Practice Paper No.2
- Practice Paper No.3
- Practice Paper No.4
