All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2008 > Chemistry
AIEEE 2008 Chemistry
Q. 33. Standard entropy of X2, Y2 and XY3 are 60, 40 and 50 JK-1mol-1, respectively. For the reaction, 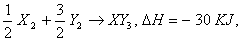 , to be at equilibrium, the temperature will be
, to be at equilibrium, the temperature will be
- 750 K
- 1000 K
- 1250 K
- 500 K
Sol:
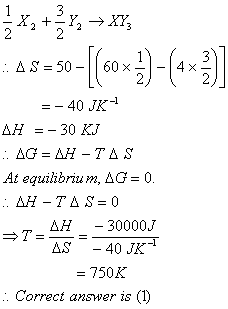
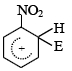
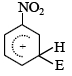
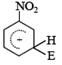
Q. 34. The electrophile, 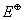 attacks the benzene ring to generate the intermediate
attacks the benzene ring to generate the intermediate 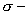 complex. Of the following, which
complex. Of the following, which 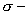 complex is of lowest energy?
complex is of lowest energy?
Sol: 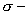 complex formed by the attack of electrophile on benzene is the more stable than that formed by the attack of electrophile on nitrobenzene at any one of the three given positions.
complex formed by the attack of electrophile on benzene is the more stable than that formed by the attack of electrophile on nitrobenzene at any one of the three given positions.
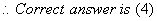
Q. 35. 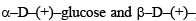 Glucose are
Glucose are
- anomers
- enantiomers
- conformers
- epimers
Sol: