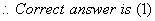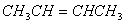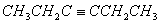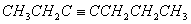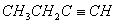All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2008 > Chemistry
AIEEE 2008 Chemistry
Q. 5.The hydrocarbon which can react with sodium in liquid ammonia is
Sol:
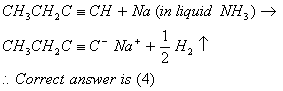
Q. 6.
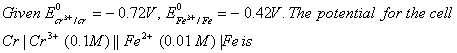
- -0.339 V
- -0.26 V
- 0.26 V
- 0.339 V
Sol:
Q. 7. Amount of oxalic acid present in a solution can be determined by its titration with KMnO4solution in the presence of H2SO4. The titration gives unsatisfactory result when carried out in the presence of HCl, because HCl
- reduces permanganate to Mn2+.
- oxidises oxalic acid to carbon dioxide and water.
- gets oxidised by oxalic acid to chlorine.
- furnishes H+ ions in addition to those from oxalic acid.
Sol: KMnO4can oxidise HCl along with oxalic acid into Cl2 and itself gets reduced to Mn2+.