![]()
CBSE Guess > Papers > Important Questions > Class X > 2009 > Science > Science By Mr. Pravin Kumar
CBSE CLASS X
Section A
Q. 1. Give the chemical formula of rust.
Q. 2. Plaster of Paris should be stored in a moisture-proof container. Explain why?
Q. 3. From which alcohol and acid ethyl ethanoate supposed to be formed. Give molecular formula of each.
Q. 4. An organic compound burns with a sooty flame. Is it a saturated or an un saturated compound?
Q. 5. What would be the electron dot structure of carbon dioxide which has the formula CO2?
Q. 6. Why potato chips manufacturers fill the packet of chips with nitrogen gas?
Q. 7. A student has been collecting silver coins and copper coins. One day she observed a black coating on silver coins and a green coating on copper coins. Which chemical phenomenon is responsible for these coatings? Write the chemical name of black and green coatings.
Q. 8. Dry Hydrogen Chloride gas does not turn blue litmus red whereas Hydrochloric acid does. Give one reason.
Q. 9. Identify the substance oxidised and reduced in the chemical reaction :
Mn O2 + 4 HCl ——> Mn Cl2 + Cl2 +2H2O
Q. 10. What is IUPAC name of Acetic acid and ethyl alcohol.
Q. 11. What is meant by denatured alcohol?
Q. 12. Why should curd and sour substances not be kept in brass and copper vessels?
Q. 13. What is the common name of the compound CaOCl2?
Section B
Q. 14. A housewife wanted her house to be whitewashed. She bought 10kg of quick lime from the market and dissolved it in 30 litres of water. On adding lime to water she noticed that the water started boiling even when it was not being heated. Give reason for her observation. Write the corresponding chemical equation and name the product formed.
Q. 15. How does atomic size of elements vary on moving from
(i) left to right in a period.
(ii) from top to bottom in a group.
Give reasons for your answers.
Q. 16. What is the difference between roasting and calcinations? Which one of two is used for sulphide ores?
Q. 17. Explain how the following metals are obtained from their compounds by the reduction process:
- Metal x, which is low in the reactivity series.
- Metal y, which is in middle the reactivity series.
- Metal z, which is high up in the reactivity series.
Q. 18. A solution of a substance ‘X’ is used for white washing.
- Name the substance ‘X’ and write its formula.
- Write the reaction of the substance ‘X’ named in (i) above with water.
Q. 19. Write the Common name and IUPAC name for the following compounds:
- CH3 – CH2 - CH2 – Cl
- CH3 – CH2 - CH2 – OHj
Section C
Q. 20. Define homologues series? What are the characteristic properties of homologues series? Explain with an example?
Q. 21. Write word equations and then balanced equations for the reaction taking place when –
- Dilute sulphuric acid reacts with zinc granules.
- Dilute hydrochloric acid reacts with magnesium ribbon.
- Dilute sulphuric acid reacts with aluminium powder.
- Dilute hydrochloric acid reacts with iron filings.
Q. 22. Give reasons
- Platinum, gold and silver are used to make jewellery.
- Sodium, potassium and lithium are stored under oil.
- Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
- Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Q. 23. Write the balanced equation for the following chemical reactions.
- Hydrogen + Chlorine → Hydrogen chloride
- Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
- Sodium + Water → Sodium hydroxide + Hydrogen
Q. 24. The following table shows the position of six elements A, B, C, D, E and F in the periodic table.
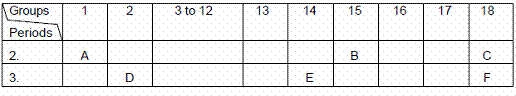
Using the above table answer the following questions:
- Which element will form only covalent compounds?
- Which element is a metal with valency 2?
- Which element is a non-metal with valency of 3?
- Out of D and E, which one has a bigger atomic radius and why?
- Write a common name for the family of elements C and F.
Q. 25. An organic compound ‘A’ is widely used as a preservative in pickles and has a molecular formula C2H2O2. This compound reacts with ethanol to form a sweet smelling compound ‘B.
- Identify the compound ‘A’
- Write the chemical equation for its reaction with ethanol to form compound ‘B’.
- How can we get compound ‘A’ back from ‘B’?
- Name the process and write corresponding chemical equation.
- Which gas is produced when compound ‘A’ reacts with washing soda? write the chemical equation.
Q. 26.
- Why does carbon form largest number of compounds?
- Why are some of these called saturated and other unsaturated compounds?
- Which of these two is more reactive?
- Write the names of the compounds.

Q. 27. Two carbon compounds A and B have the molecular formula C3 H8 and C3 H6 respectively. Which one of the two is most likely to show addition reaction? Justify your answer. Explain with the help of a chemical equation, how an addition reaction is useful in vegetable ghee industry.
Q. 28. Give an example of a metal which
- is a liquid at room temperature.
- can be easily cut with a knife.
- is the best conductor of heat.
- is a poor conductor of heat.
Section D
Q. 29. Baking soda is used in small amount in making bread and cake. It helps to make these soft and spongy. An aqueous solution of baking soda turns red litmus blue. It is also used in soda acid fire extinguisher.
Use this information to answer the following questions:
- How does Baking Soda help to make cakes and bread soft and spongy?
- How does it help in extinguishing fire?
- is the pH value of baking soda solution lesser than or greater than 7?
Q. 30.
- What were the two achievements of Mendeleev’s Periodic table? What was the basis of classification of elements in it?
- An element X (2, 8, 2) combines separately with NO3 and (SO4)2-, (PO4)3- radicals. Write the formulae of the three compounds so formed. To which group of the periodic table does the element ‘X’ belong? Will it form covalent or ionic compound? Why?
Paper By Mr. Pravin Kumar
E mail [email protected]
