All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2007 > Chemistry
AIEEE 2007 Chemistry
Q. 37. The density (in g mL–1) of a 3.60 M sulphuric acid solution that is 29%
H2SO4 (Molar mass = 98 g mol–1) by mass will be
- .64
- 1.88
- 1.22
- 1.45
Sol: Let the mass of solution be x g.
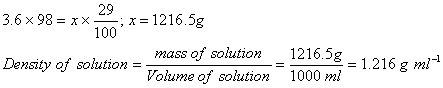
Correct choice: (3)
Q. 38. The first and second dissociation constants of an acid H2A are 1.0 × 10–5 and 5.0 × 10–10 respectively. The overall dissociation constant of the acid will be
- 5.0 × 10–5
- 5.0 × 1015
- 5.0 × 10–15
- 0.2 × 105
Sol: ![]()
Correct choice: (3)
Q. 39. A mixture of ethyl alcohol and propyl alcohol as vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be
- 350
- 300
- 700
- 360
Sol:
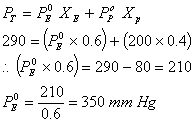 Correct choice: (1)
Correct choice: (1)
Q. 40. In conversion of lime–stone to lime, ![]()
The values of ![]() and
and ![]() are +179.1 kJ mol–1 and 160.2 J/K respectively at 298 K and 1 bar. Assuming that
are +179.1 kJ mol–1 and 160.2 J/K respectively at 298 K and 1 bar. Assuming that ![]() and
and ![]() do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is
do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is
- 1008 K
- 1200 K
- 845 K
- 1118 K
Sol: ![]() The decomposition of CaCO3 to CaO and CO2 would become spontaneous when
The decomposition of CaCO3 to CaO and CO2 would become spontaneous when ![]() would be –ve. But limiting condition can be arrived at when
would be –ve. But limiting condition can be arrived at when ![]() would become zero.
would become zero. 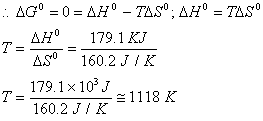
Correct choice: (4)