All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2007 > Chemistry
AIEEE 2007 Chemistry
Q. 28. Which of the following nuclear reactions will generate an isotope?
- neutron particle emission
- positron emission
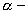 particle emission
particle emission 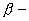 particle emission
particle emission
Sol: In a nuclear reaction, an isotope cannot be generated by the emission of positron ![]() , particle or
, particle or ![]() particle because it will change the atomic number of the parent nuclei. An isotope can be generated when the nuclei of an element are bombarded with slow neutrons e.g.,
particle because it will change the atomic number of the parent nuclei. An isotope can be generated when the nuclei of an element are bombarded with slow neutrons e.g., ![]() . Formation of isotope by neutron particle emission is a hypothetical process.
Correct choice: (1)
. Formation of isotope by neutron particle emission is a hypothetical process.
Correct choice: (1)
Q. 29. The equivalent conductances of two strong electrolytes at infinite dilution in H2O (where ions move freely through a solution) at 250C are given below: ![]()
What additional information/quantity one needs to calculate of an aqueous solution of acetic acid?
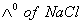
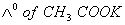
- (C) The limiting equivalent conducted of
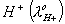
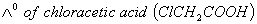
Sol:
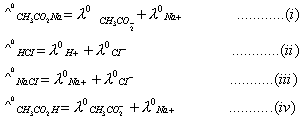 Correct choice: (1)
Correct choice: (1)
Q. 30. Which one of the following is the strongest base in aqueous solution?
- Trimethylamine
- Aniline
- Dimethylamine
- Methylamine
Sol: Aliphatic amines are more basic than aromatic amines and among aliphatic amines, the order of basicity in aqueous solution is ![]() Correct choice: (3)
Correct choice: (3)
Q. 31. The compound formed as a result of oxidation of ethyl benzene by KMnO4 is
- benzophenone
- acetophenone
- benzoic acid
- benzyl alcohol
Sol:
![]() Correct choice: (3)
Correct choice: (3)