All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2007 > Chemistry
AIEEE 2007 Chemistry
Q. 4. Consider the reaction, ![]() When concentration of B alone was doubled, the half−life did not change. When the concentration of A alone was doubled, the rate increased by two times. The unit of rate constant for this reaction is
When concentration of B alone was doubled, the half−life did not change. When the concentration of A alone was doubled, the rate increased by two times. The unit of rate constant for this reaction is
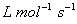
- no unit
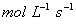
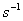
Sol: The reaction would be first order with respect to A and first order with respect to B. The overall order of the reaction is 2.
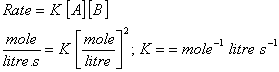
Correct choice: (1)
Q. 5.. Identify the incorrect statement among the following:
- d−Block elements show irregular and erratic chemical properties among themselves .
- La and Lu have partially filled d orbitals and no other partially filled orbitals.
- The chemistry of various lanthanoids is very similar.
- 4f and 5f orbitals are equally shielded.
Sol: 4f orbital electrons are shielded more than 5f orbital electrons.
Correct choice: (4)
Q. 6. Which one of the following has a square planar geometry?
(Atomic numbers: Co = 27, Ni = 28, Fe = 26, Pt = 78)
Sol: Platinum(+II) only forms square planar complex. Correct choice: (4)
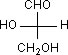
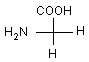
Q. 7. Which of the following molecules is expected to rotate the plane of plane − polarised light?
Sol: Compound (1) does not have any plane of symmetry as well as centre of symmetry so it rotates the plane of plane−polarised light. Compound (2), (3) and (4) have a plane of symmetry, so they all are optically inactive.
Correct choice: (1)