
CBSE Guess > Papers > Question Papers > Class X > 2009 > Science > Science
 
SCIENCE - 2009
Comptt
(Set - III Outside Delhi )
Time allowed : 2 1/2 hours
Maximum marks : 60
General Instructions :
(i) The question paper comprises of two Sections, A and B. You are to attempt both
the Sections.
(ii) All questions are compulsory.
(iii) There is no overall choice. However, internal choice has been provided in all the
three questions of five marks category. Only one option in such questions is to be
attempted.
(iv) All questions of Section A and all questions of Section B are to be attempted
separately.
(v) Questions number 1 to 6 in Section A and 17 to 19 in Section B are short answer
type questions. These questions carry one mark each.
(vi) Questions number 7 to 10 in Section A and 20 to 24 in Section B are short answer
type questions and carry two marks each.
(vii) Questions number 11 to 14 in Section A and 25 and 26 in Section B are also short
answer type questions and carry three marks each.
(viii) Questions number 15 and 16 in Section A and question number 27 in Section B
are long answer type questions and carry five marks each.
Section A
Q.1. Why was the system of classification of elements into triads not found suitable? 1
Ans. The system of classification of elements into triads was not found suitable because it failed to arrange all the then known elements in the form of triads of elements having similar chemical properties. Only three triads from the elements known at that time could be identified.
Q. 2. What is a homologous series? 1
Ans. A homologous series is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by CH2 group.
Q. 3. State the first limitation of Mendeleev’s periodic table. 1
Ans. Limitation of Mendeleev’s periodic table:
- The position of isotopes could not be explained as isotopes are the atoms of the same element having similar chemical properties but different atomic masses.
Q. 4. Draw the structure of ethanol molecule.
Ans. Ethanol C2H5OH
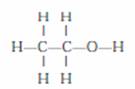
 
CBSE 2009 Question Papers Class X
|