SCIENCE AND TECHNOLOGY— 2006 (Set II — Outside Delhi)
SECTION A
Q. 1. A ray of light is incident on a convex mirror as shown on.
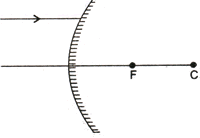
Redraw the diagram after completing the path of the light ray after reflection from the mirror. (1)
Q. 2. How can you show that the magnetic field produced by a given electric current in the wire decreases as the distance from the wire increases? (1)
Q. 3. Name the product other than water formed on burning of ethanol in air.( 1)
Q. 4. Why does magnesium powder react much more rapidly than magnesium ribbon with dilute sulphuric acid? (1)
Q. 5. Name the type of nuclear reaction by which the Sun produces its energy. List two conditions which are present at the center of the sunresponsible for this reaction.
Q. 6. Given below are the pH values of four different liquids:
7.0, 14.0, 4.0, 2.0
Which of these could be that of:
- lemon juice,
- distilled water,
- 1 M sodium hydroxide solution,
- tomato juice? (2)
Q. 7. An object is placed at a distance of 12cm in front of a concave mirror. It forms a real image four times larger than the object. Calculate the distance of the image from the mirror. (2)
Q. 8.
- Draw a diagram to show how two resistors R1 and R2 are connected in series.
- In a circuit if the two resistors of 5 ohm and 10 ohm are connected in series, how does the current passing through the two resistors compare? (2)
Or
A bulb is rated at 5.0 volt, 100 mA. Calculate its
- power and
- resistance.
Q. 9.
- How is methanal obtained from methanol?
- Write the chemical equation of the reaction involved in the preparation of methanal from methanol
- Mention one use of an aqueous solution of methanal in the biology laboratory. (3)
Or
Complete the following reaction equations:
- CH3CH2OH
- HCHO + H2
- CH3COOH + C2H5OH
Q. 10.
- Name the four gases commonly present in biogas.
- List two advantages of using biogas over fossil fuels. (3)
Q. 11.
- Name the raw materials used in the manufacture of sodium carbonate by Solvay process.
- How is the sodium hydrogen carbonate formed during Solvay process separated from a mixture of NH4CI and NaHCO3?
- How is sodium carbonate obtained from sodium hydrogen carbonate? (3)
Q. 12.
- What is the action on litmus of:
- Dry ammonia gas
- Solution of ammonia gas in water.
- State the observations you would make on adding ammonium hydroxide to aqueous solutions of:
- Ferrous sulphate
- Aluminium chloride.
Or
Explain the following terms by giving one example of each: (3 )
- Mineral
- Ore
- Gangue.
Q. 13. What is a nuclear reactor? State one function each of
- coolant and
- moderator in a nuclear reactor. (3)
Q. 14. Distinguish between natural and artificial satellites.
To launch an artificial satellite in an orbit around the Earth what is the minimum:
- Horizontal velocity required for the push?
- Height to lift the satellite from the ground? (3)
Q. 15.
- What is an electromagnet? What does it consist of?
- Name one material in each case which is used to make a
- Permanent magnet
- Temporary magnet.
- Describe an activity to show how you can make an electromagnet in your school laboratory. (5)
Q. 16. What is an alloy? How is an alloy made? List two purposes of making alloys. Mention the constituents and two properties of each of the following alloys: (5)
- Stainless steel
- Brass.
SECTION - B
Q. 17. Write the expanded form of AIDS. (1)
Q. 18. Name the type of fission carried out by Amoeba. (1)
Q. 19. “Rapid increase of population disturbs the biotic environment” Justify this statement taking any two aspects. (2)
Q. 20. Differentiate between tropic and nastic movements in plants. Give one example of each. (2)
Q. 21. Write the functions of the following in the digestive process: (3)
- Bile
- Bicarbonate secreted by the duodenal wall
- Pancreatic amylase.
Q. 22. Give reasons for the following: (3)
- The glottis is guarded by epiglottis.
- The lung alveoli are covered with blood capillaries.
- The wall of trachea is supported by cartilage rings.
Q. 23. Who proposed the “Theory of Natural Selection”? Explain this theory briefly. (3)
Q. 24.
- Draw a diagram of the human urinary system and label in it:
- Kidney
- Ureter
- Urinary bladder
- Urethra.
- Name the two major components of normal human urine. (5)
Or
- Name the blood groups under ABO system.
- Differentiate between universal donor and universal recipient under this system. (5)
English 2006 Question Papers Class X
CBSE 2006 Question Papers Class X
