SECTION - A
Q. 1. Name the largest component of biogas. 1
Q. 2. Name the metal used for making the body of an aircraft. 1
Q. 3. Name the factors which affect the rate of reaction. 1
Q. 4. Name the two components of baking powder. 1
Q. 5. If the magnification of a body of size 1m is 2, what is the size of the image? 1
Q. 6. Write four characteristics used for selecting a suitable fuel. 2
Or
What is a solar cell? Name two materials mostly used for making solar cells. 2
Q. 7. What is nuclear fusion? Give an example for it. 2
Q. 8. For producing electricity, the energy from flowing water is preferred to energy obtained by burning coke. State two reasons for it. 2
Q. 9. State the rule to determine the direction of magnetic field produced around a current carrying conductor. 2
Q. 10. How is neoprene obtained? Why is it considered superior to natural rubber? 2
Q. 11. Three resistors are connected as shown in the figure. Through a resistor 5 ohm a current of one ampere is flowing: 3
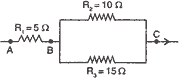
(a) what is the p.d. across AB and across AC?
(b) what is the current through the other two resistors?
(c) what is the total resistance?
Q. 12. An organic compound X is an essential constituent of wine and beer. X is responsible for the intoxication caused by these drinks. Oxidation of X yields an organic acid Y which is present in vinegar. Name the compounds X and Y and write their structural formulae. 3
Or
State two reasons for counting sulphur amongst the non-metals. Which properties of sulphur make it possible to bring it from a deep mine to the surface of the earth by the Frasch process and to purify it by the sublimation of liquid sulphur? 3
Q. 13. List four essential conditions for life to originate and flourish on a planet. Why do we not expect existence of life on the planet Mars? 3
Q. 14. For the reaction
2SO2 (g) + O2 (g) -->2SO3(g)
at equilibrium at 1000 K, the molar concentrations of SO2, 02, SO3 are 1.44, 1.98 and 0.41 respectively. Calculate the equilibrium constant for this reaction. 3
Q. 15. What is meant by quenching, annealing and tempering of steel? 3
Q. 16. Draw a ray diagram of a microscope 3
Q. 17. Differentiate between soap and detergent. 3
Q. 18. Draw a labelled diagram of solar cooker. What purposes are served by the blackened surface, glass cover plate and the mirror in a solar cooker? What would happen if the plain glass mirror of a solar cooker is replaced by a concave glass mirror? 5
Q. 19. Why are stars not the permanent objects of the universe? Give various stages in the life of a star. 5
Or
What will happen when:
(a) ethanoic acid reacts with sodium carbonate?
(b) propanone reacts with hydrogen cyanide?
(c) ethanol is heated with alkaline potassium permanganate?
(d) methanal reacts with hydrogen cyanide?
(e) ethanal reacts with Fehling’s reagent? 5
Q. 20. Describe Haber process for the manufacture of ammonia. Give the role of promoter in this process. Draw a flow diagram of this process. 5
Or
Give the effect of heat on sulphur. Explain with the help of diagrams. 5
SECTION - B
Q. 21. What is glycolysis? 1
Q. 22. Define translocation. 1
Q. 23. What is dialysis? 1
Q. 24. How electrostatic precipitators help in controlling particulate emissions? 2
Q. 25. Differentiate between menarche and menopause. 2
Q. 26. What is eutrophication? Mention its harmful effects. 3
Q. 27. Show in tabular form the different blood transfusions that should occur among the various blood groups in human beings. 3
Q. 28. What is genetic engineering? Give the full form of GMO. 3
Or
Differentiate between autosomes and sex chromosomes.
Q. 29. Define reflex action with suitable examples. 3
Q. 30. Explain the human digestive system with the help of a diagram. 5
Or
Describe Darwin’s theory of Evolution. 5