![]()
CBSE Guess > Papers > Important Questions > Class XII > 2013 > Chemistry > Solid State By Mr. R. Srinivas Vasudevamurthy
CBSE CLASS XII
Surface Chemistry - 4 Marks Questions
1. Give differences between
a) adsorption and absorption
b) physisorption and chemisorption
c) lyophillic and lyophobic sol.2. Explain the terms
a) extent of adsorption(x/m)
b) enthalpy of adsorption
c) desorption
d) adsorbent
e) adsorbate3. Mention the factors affecting the adsorption of gases on solids.
4. Explain Freundlich adsorption isotherm with suitable graphs.
5. Why is adsorption
a) exothermic process
b) surface property6. Explain the effect of temperature on physisorption and chemisorption with graphs.
7. Mention five applications of adsorption.
8. Explain activity and selectivity of a catalyst with suitable examples.
9. What are shape selective catalysts? Give one example.
10. Give three examples each of homogeneous and heterogeneous catalysis.
11. Explain the mechanism of
a) homogeneous catalysis
b) heterogeneous catalysis.12. Give three examples of reactions involving enzyme as catalyst. Explain the mechanism of enzyme catalysed reaction.
13. Mention the characteristics of enzyme catalysis.
14. Mention the disperse phase and dispersion medium in
a) Milk
b) butter
c) cheese
d) Fog
e) mist
f) froth15. What do you mean by multi molecular and macro molecular colloids? Give two examples each.
16. What are associated colloids? How does it differ from multi molecular and macro molecular colloids? Give two examples of associated colloids.
17. Explain the terms
a) kraft temperature
b) CMC(critical micelle concentration)18. Explain the mechanism of cleaning action of soap.
19. Action of soap is due to emulsification and micelle formation. Comment.
20. How is sulphur sol prepared by chemical method?
21. How is gold sol prepared by
a) chemical method
b) Bredig’s arc method?22. Mention the principle of dialysis.
23. What is Tyndall effect? Mention the condition for Tyndall effect to occur. Give two application of Tyndall effect.
24. What is Brownian movement? Mention the significance of Brownian movement
25. Describe and explain what is observed when a) beam of light is passed through
a) colloidal solution.
b) an electric current is passed through a colloidal solution.26. Account for the following:
a) lyophillic colloids are more stable than lyophobic colloids.
b) delta formation occurs at the river beds.
c) alum or ferric chloride is added to stop bleeding.
d) sky appears blue in colour.
e) alum is used in water purification.27. Explain the terms
a) zeta potential
b) Helmoltz electrical double layer
c) coagulation
d) Colloidion
e) peptisation28. Explain Hardy and Schulze rule with suitable examples.
29. What are emulsions? How are they classified? Give two examples each.
30. Explain the terms
a) emulsification
b) demulsification
c) emulsifier.31. Write two uses of emulsion.
32. List four applications of colloids.
33. What is coagulation value?
34. Out of ammonia and nitrogen which gas will be absorbed more readily on the surface of Charcoal and why?
35. How does adsorption of a gas vary with
a) temperature
b) pressure? Illustrate with graphs.36. Describe some features of catalysis by zeloite. Mention one application of zeolite.
37. What do you mean by activation of adsorbent? How is it achieved?
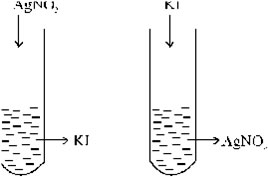
38. A colloidal solution of AgI is prepared by two different methods shown below:-
(i) What is the charge of AgI colloidal particles in the two test tubes (A) and (B)?
(ii) Give reasons for the origin of charge.
Submitted By : Mr. R. Srinivas Vasudevamurthy
Email: [email protected]