All India Engineering / Architecture Entrance Examination (AIEEE)
CBSE Guess > AIEEE > AIEEE Papers > 2009 > Chemistry
AIEEE 2009 Chemistry
Q. 21. The IUPAC name of neopentane is:
- 2,2–dimethylbutane
- 2–methylbutane
- 2,2-dimethylpropane
- 2–methylpropane
Sol.:
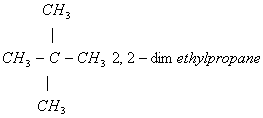
Answer: (3)
Q. 22. In context with the transition elements, which of the following statements is incorrect?
- Once the d5 configuration is exceeded, the tendency to involve all the 3d electrons in bonding decreases.
- In addition to the normal oxidation states, the zero oxidation state is also shown by these elements in complexes.
- In the highest oxidation states, the transition metal show basic character and form cationic complexes.
- In the highest oxidation states of the first five transition elements (Sc to Mn), all the 4s and 3d electrons are used for bonding.
Sol.: In highest oxidation state, the transition metal will form complexes which will have large degree of covalent character, which shows the acidic behaviour.
Answer: (3)
Q. 23. Which of the following pairs represents linkage isomers?
- [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2
- [Cu(NH3)4][PtCl4] and [Pt(NH3)4] [CuCl4]
- [Pd(PPh3)2(NCS)2] and [Pd(PPh3)2(SCN)2]
- [Co(NH3)5NO3]SO4 and [Co(NH3)5SO4]NO3
Sol: Linkage isomerism is shown by ambidentate ligands. (SCN– and NCS– are ambidentate groups).
Answer: (3)